
The universe in its totality might be considered a closedsystem of this type this would allow the two laws to be applied to it.


DOI: 10.1103/ and the Laws of Thermodynamics The principal energy laws that govern every organization are derivedfrom two famous laws of thermodynamics. Entropy production given constraints on the energy functions. “There’s this deep relationship between physics and information theory.”
ENTROPY LAW OF THERMODYNAMICS HOW TO
Kolchinsky said, “A better understanding of mismatch cost could lead to a better understanding of how to predict and correct those errors.” They also discovered that this fundamental relationship extends even more broadly than previously believed. This way, they could strengthen the second law of thermodynamics. They’ve discovered a fundamental relationship between thermodynamic irreversibility-the case in which entropy increases - and logical irreversibility - the case in computation in which the initial state is lost. In previous studies, scientists demonstrated that this mismatch cost could be found in several systems, not just in information theory. “It’s the cost between what the machine is built for and what you use it for.” The researchers call this a “mismatch cost,” or the cost of being wrong.” Kolchinsky and Wolpert have demonstrated that two computers might carry out the same calculation, for example, but differ in entropy production because of their expectations for inputs. If the computer carries out some other calculation, then it will generate additional entropy.
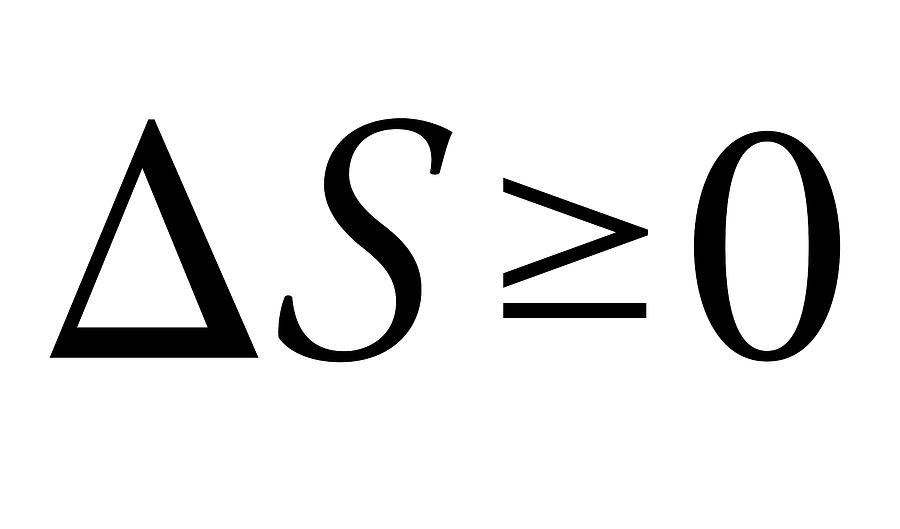
Kolchinsky said, “The only way to achieve Landauer’s minimum loss of energy is to design a computer with a certain task in mind. In this study, the SFI duo found that most systems produce more. Landauer derived an equation for the minimum amount of energy that is produced during erasure. Scientists wanted to determine the energy cost of erasing information for a given system. In 1961, IBM physicist Rolf Landauer discovered that when information is erased, as during such a calculation, the entropy of the calculator decreases (by losing information), which means the environment’s entropy must increase.Įrasing a bit of information requires generating a little bit of heat. Just from the answer, the machine can’t report which pair of numbers acted as input. While generating the output, the machine loses information about input as the same output can be obtained by 3+1. The addition of 2 and 2 gives output as 4 in the calculator.
Wolpert said, “Computing systems are designed specifically to lose information about their past as they evolve.” To better understand the connection between thermodynamics and information processing in computation, physicist and SFI resident faculty member David Wolpert in collaboration with Artemy Kolchinsky, physicist and former SFI postdoctoral fellow, conducted a new study in which they look at applying these ideas to a wide range of classical and quantum areas, including quantum thermodynamics.


 0 kommentar(er)
0 kommentar(er)
